Lab hacks 🧬
I created this particular page as a personal space for documenting essential knowledge in any lab setting 📝 . To me, understanding what you are doing is always way more important than following the protocol without any consideration. I found that there are some small details in the lab that you are so familiar with that you almost forget their purpose 🚩. For example, I was once very disappointed with myself when I confused the use of SYBR Safe when running PCR, a universal technique that every single lab is using. Therefore, I hope these insights, once organized, will help me to reinforce my understanding for my own good; and it would be amazing if this resource is useful for your studies as well, especially for biomed freshers or undergrad students ✨
Gel electrophoresis 🧪
What is TAE buffer?
Tris - Acetic Acid - EDTA buffer is the one used for running agarose gel electrophoresis. The pH of the TAE buffer is higher than the neutral (pH = 8.3)
What does EDTA do?
EDTA is the chelating reagent that will help to chelate the magnesium ions bound on the nuclease, which can degrade DNA. EDTA can accomplish this role because it can be chelated with Mg2+ and Ca2+ under alkaline conditions (pH>9). Therefore, EDTA in the buffer will help to prevent DNA degradation from the nuclease.
What is the DNA stain?
DNA stain is a sensitive fluorescent reagent used to detect nucleic acid. It is added to the TAE buffer when running the gel. What is the difference between SYBR Safe and EtBr?
Both are known as DNA stains used to detect nucleic acid, but SYBR Safe is safer than EtBr (Ethidium Bromide), which is quite controversial regarding their carcinogenicity. However, SYBR Safe is also not completely safe for users, so we need to handle it with gloves every time. The gel containing SYBR Safe also needs to be discarded in a specified container.
Western blot 🎀
What is NP40 buffer?
NP40 buffer prepares the extracts from cells and tissues for WB, IP, and ELISA experiments. NP40 itself is a non-ionic detergent that helps to disrupt the lipid bilayer membrane and solubilize the contents inside the cells into homogenous extracts.
The composition of NP40 is as follow: 25 mM Tris-HCl, pH 7.4, 150 mM NaCl, 10% glycerol, and 1% NP-40.
What is the difference between NP40 and RIPA buffer?
NP40 is a non-ionic detergent composed of Tris, H20, and NP40. However, RIPA is composed of more complex ingredients, including some ionic compounds (NaCl and anionic SDS), which makes them generally harsher than NP40.
NP40 is preferred when preserving the protein-protein interaction, especially for IP experiments. On the other hand, RIPA fosters a more comprehensive cell lysis, which disrupts the cell membrane and the nuclear membrane to extract both the cytoplasmic and nuclear protein.
What is TBST buffer? What does Tween 20 do?
TBS stands for Tris-Buffered Saline, commonly known as a washing buffer.
TBST = TBS + Tween 20. This buffer is used for maintaining pH and increasing signal-to-noise ratio, which enhances the washing efficacy
What is BSA? What is the difference between BSA with 5% non-fat milk?
BSA (Bovine Serum Albumin) only contains one pure protein, so it is highly consistent in composition and harder to degrade, so it is preferable to use it to prepare the primary antibody.
Non-fat milk contains a mixture of various proteins, so it works very well for preventing unspecific binding.
Why do we need Methanol to activate the PVDF membrane?
When the membrane is exposed to methanol, it undergoes a slight swelling, which can increase the pore size of the membrane. This porosity allows for better access of proteins into the membrane structure, facilitating effective binding. Why do we need Methanol in the transfer buffer?
To sharpen the protein band and reduce the swelling of the gel during the transfer process because the gel can get swollen when exposed to the electric current, which can cause incomplete transfer.
Methanol can fulfill this responsibility because it is a polar solvent that can interact with water molecules in the gel.
This interaction will dehydrate the gel and make it more compact. Moreover, by removing the water from the polyacrylamide gel, the pore size of the gel will be lowered, reducing the risk of being distorted.
Cell culture 🧫
What is FBS? Why do we need to add it to the culture medium? And why do we need to use it in a freezing medium?
FBS is Fetal Bovine Serum. It is added to the culture media to supply nutrients and growth factors
FBS has a cryoprotective characteristic to protect the cells from the freezing and thawing process, which is pretty harsh for them due to the formation of ice crystals that can damage the cell membrane.
Why do we need to add Glutamine but not any other amino acid to the cell culture medium?
Glutamine is the important substrate of the TCA cycle that can provide an energy source (ATP production)
Glutamine acts as an essential nitrogen donor to synthesize other amino acids. For example, glutamine donates nitrogen to become glutamate through the deamination process
L-Glutamine = L-alanyl-L-glutamine (dipeptide form of glutamine)
Why is 70% ethanol used in the cell culture lab but not 90% or 100%?
70% Ethanol is the optimal condition, which is more diluted than complete ethanol, making it easier for the ethanol molecules to penetrate and attack the bacterial cell wall. Also, the higher concentrated ethanol will evaporate more quickly, limiting the contact time to the microorganism.
Why do we need to add DMSO to the freezing medium?
DMSO and FBS serve as cryoprotective agents that reduce the freezing point of the medium. This allows a more sustainable cooling rate, reducing the potential damage done by ice crystal formation. The serum used is extensively tested to protect cells during cell preservation.
Why can trypsin detach cell? What is so special about them?
Trypsin is a proteolytic enzyme needed for detaching adherent cell cultures and monolayers. This globular, pancreatic protease cleaves at the C-terminal side of lysine and arginine, breaking down vessel-adhering proteins and allowing easy resuspension during cell harvesting.
qPCR 🧬
What is the difference between oligo d(T) primer and random hexamer?
Oligo d(T) binds specifically to the 3’ polyA tail, which is also the best characteristic of mRNA, so using oligo d(T) will have a better mRNA yield.
On the other hand, random hexamer can bind to various template, including rRNA and tRNA as well, so this is a better choice if you want to extract the total RNA from the lysate and when mRNA is somehow degraded.
What is the chemical rationale behind RNA extraction kits (including RNeasy Plus Mini Kit and TRIzol reagent)
The cell lysate would be denatured in guanidine-isothiocyanate (GITC)–containing buffer, which immediately inactivates RNases to ensure isolation of intact RNA. The lysate is then passed through a gDNA Eliminator spin column. This column, in combination with the optimized high-salt buffer, allows efficient removal of genomic DNA.
Firstly, GITC can disrupt the tertiary and quaternary structures of proteins.
Secondly, it helps with the chelation of Metal Ions: Some RNases require metal ions, such as Mg2+, for their catalytic activity. GITC can chelate these metal ions, preventing them from binding to the active sites of RNases and thereby inhibiting their enzymatic activity.
While GITC denatures proteins, it can also stabilize RNA molecules. This stabilization can prevent RNA degradation by RNases, as the RNA molecules become less accessible to the enzyme due to their altered structure in the presence of GITC.
Immunoprecipitation 🧸
Why except for the phosphorylation inhibitor and protease inhibitor, do we include nuclease in the lysis buffer?
To fragment the DNA pieces so we can extract more protein for solubilization.
Why do we need the input lysate for immuno-precipitation?
The lysate acts as the control for the proteins present to quantify how much of the target protein was pulled down relative to its initial abundance in the lysate.
The lysate run parallel with the IP is for ensuring that the antibodies used are specific to the target protein
Cell viability assay 💫
What is the prinicple behind Alamar blue assay?
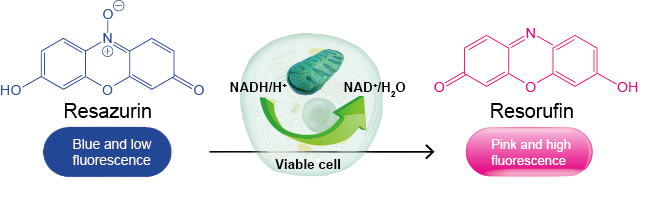
The Alamar Blue (AB) cell viability assay harnesses the optical property change associated with the conversion of resazurin (oxidized, non-fluorescent, blue) to resorufin (reduced, fluorescent, pink) that takes place under the cellular reducing environment.
The intensity of pink fluorescence is proportional to cellular metabolic activities, therefore the number of living and respiring cells.
Other reagents 📌
What is the use of MG-132?
MG-132 acts as a proteasome inhibitor. Thus, its primary function is to inhibit the proteasome, a protein complex responsible for degrading unwanted or misfolded proteins tagged with ubiquitin. By blocking the proteasome's activity, MG-132 prevents the degradation of these proteins, so they will increase their expression.
 tammychandesu
tammychandesu